NCERT Textbook Solution Page no. 3
Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, smell of perfume.
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
Question 3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show
Answer: A diver is able to cut through water in a swimming pool. This shows that the particles of water have intermolecular space and has less force of attraction.
Question 4. What are the characteristics of the particles of matter?
Answer. The characteristics of the particles of matter are:
(1) Particles have intermolecular space.
(2) Particles have intermolecular force.
(3) Particles of matter are moving continuously.
NCERT Textbook Solution Page no. 6
(density = mass/volume).
Arrange the following in order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton and iron.
air < exhaust from chimneys < cotton < water < honey < chalk < iron.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
(i) Rigidity: The tendency of a substance to retain/maintain their shape when subjected to outside force.
(ii) Compressibility: The matter has intermolecular space. The external force applied on the matter can bring these particles closer. This property is called compressibility. Gases and liquids are compressible.
(iii) Fluidity: The tendency of particles to flow is called fluidity. Liquids and gases flow.
(iv) Filling of a gas container: Gases have particles which vibrate randomly in all the directions. The gas can fill the container.
(v) Shape: Solids have maximum intermolecular force and definite shape.
Whereas liquids and gases takes the shape of container.
(vi) Kinetic energy: The energy possessed by particles due to their motion is called kinetic energy. Molecules of gases vibrate randomly as they have maximum kinetic energy.
(vii) Density: It is defined as mass per unit volume, the solids have highest density.
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
(a) The particles of gases have less force of attraction between them. The particles are free to move, so they will fill the vessel in which it is kept.
(b) The particles of gas have a weak force of attraction. So, they are constantly moving randomly. The pressure exerted by a gas is due to the random motion of particles in the gas. These particles collide with the walls of the container. These collisions apply force and hence pressure on the wall.(c) The wooden table has a definite shape and a definite volume. The particles of wood are closely packed. They do not take the shape of any container. They are rigid in nature. Hence wooden table satisfies the properties of a solid.
(d) The particles of air are very loosely bounded. They are far away from each other having a lot of space between them. Hence we can easily move our hands in the air. But in a solid block, the particles are tightly held by a strong force of attraction. So there is some or no space between them. Hence we need a karate expert.
Answer: Ice is a solid but its density is lower than water due to its structure. The molecules in ice make a cage like structure with lot of vacant spaces, this makes ice float on water.
NCERT Textbook Solution Page no. 9
(a) 300 K (b) 573 K
(a) 250°C (b) 100°C
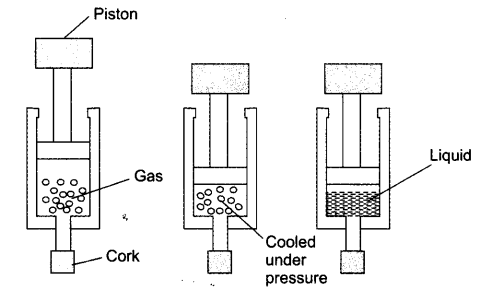
NCERT Textbook Solution Page no. 10
Question 1. Why does a desert cooler cool better on a hot dry day?
Answer: The outer walls of the cooler get sprinkled by water constantly. This water evaporates due to hot dry weather. Evaporation causes cooling of inside air of cooler. This cool air is sent in the room by the fan.
Question 2. How does the water kept in an earthen pot (matka) become cool during summer?
Answer: The earthen pot is porous with lot of pores on it, the water oozes out through these pores and the water gets evaporated at the surface of the pot thereby causing cooling effect. This makes the pot cold and the water inside the pot cools by this process.
Question 3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Answer: Acetone, petrol or perfume evaporate when they come into contact with air. The evaporation causes cooling sensation in our hands.
Question 4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Answer: Tea in a saucer has larger surface area than in a cup. The rate of evaporation is faster with increased surface area. The cooling of tea in saucer takes place sooner than in a cup. Hence we are able to sip hot tea or milk faster from a saucer rather than a cup.
Question 5. What type of clothes should we wear in summer
Answe: We should wear light coloured cotton clothes in summer. Light colour because it reflects heat. Cotton clothes because it has pores in it, which absorbs sweat and allows the sweat to evaporate faster thereby giving cooling effect.
NCERT Textbook Questions For Class 9
Question 1. Convert the following temperatures to the Celsius scale.
(a) 293 K (b) 470 K.
Answer: (a) 293 K into °C
293 – 273 = 20°C
(b) 470 K into °C 470 – 273 = 197°C
Question 2. Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C.
Answer: (a) 25°C into K
25 + 273 = 298 K
(b) 373°C into K 4 373 + 273 = 646 K
Question 3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Answer: (a) Naphthalene balls disappear with time without leaving any solid, because naphthalene balls sublime and directly changes into vapour state without leaving any solid.
(b) We can get the smell of perfume sitting several metres away because perfume contain volatile solvent and diffuse faster and can reach people sitting several metres away.
Question 4. Arrange the following substances in increasing order of forces of attraction between the particles—water, sugar, oxygen
Answer: Oxygen —> water —> sugar.
Question 5. What is the physical state of water at—
(a) 25°C (bj 0°C (cj 100°C
Answer: (a) 25°C is liquid (b) 0°C is solid or liquid
(c) 100°C is liquid and gas
Question 6. Give two reasons to justify
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
Answer: (a) Water at room temperature is a liquid because its freezing point is 0°C and boiling point is 100°C.
(b) An iron almirah is a solid at room temperature because melting point of iron is higher than room temperature.
Question 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer: Ice at 273 K will absorb heat energy or latent heat from the medium to overcome the fusion to become water. Hence the cooling effect of ice is more than the water at same temperature because water does not absorb this extra heat from the medium.
Question 8. What produces more severe bums, boiling water or steam?
Answer: Steam at 100°C will produce more severe bums as extra heat is hidden in it called latent heat whereas the boiling water does not have this hidden heat.
Question 9. Name A, B, C, D, E and F in the following diagram showing change in its state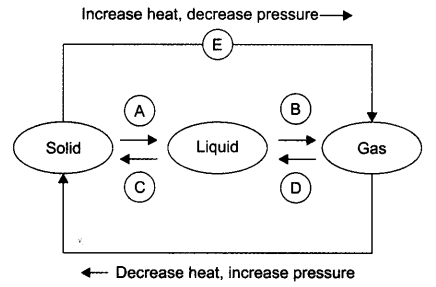
Answer: A —> Liquefication/melting/fusion B —> Vapourisation/evaporation C—>Condensation D—> Solidification E —> Sublimation F —> Sublimation
Important Question Of Matter In our Surroundings Class 9
(MCQs)
Which of the following is not a physical property of matter?
a) Boiling point
b) Melting point
c) Density
d) Chemical composition
Answer: d) Chemical compositionWhich of the following is a measure of the amount of matter in an object?
a) Mass
b) Volume
c) Density
d) Temperature
Answer: a) MassThe SI unit of density is:
a) kilogram per square meter
b) kilogram per cubic meter
c) gram per square meter
d) gram per cubic centimeter
Answer: b) kilogram per cubic meterThe state of matter that has a definite shape and volume is:
a) Solid
b) Liquid
c) Gas
d) Plasma
Answer: a) SolidWhen a liquid is heated, its particles gain:
a) Potential energy
b) Kinetic energy
c) Both potential and kinetic energy
d) Neither potential nor kinetic energy
Answer: c) Both potential and kinetic energyWhich of the following is an example of a physical change?
a) Burning of paper
b) Rusting of iron
c) Melting of ice
d) Digestion of food
Answer: c) Melting of iceThe process of change of a liquid to a gas is called:
a) Evaporation
b) Condensation
c) Sublimation
d) None of the above
Answer: a) EvaporationThe pressure exerted by a gas on the walls of the container is called:
a) Atmospheric pressure
b) Vacuum pressure
c) Gas pressure
d) Liquid pressure
Answer: c) Gas pressureThe relative motion of particles in a liquid is:
a) Vibration
b) Rotation
c) Random
d) Translational
Answer: c) RandomThe property of matter that allows it to be compressed is called:
a) Compressibility
b) Rigidity
c) Fluidity
d) Density
Answer: a) Compressibility
The process of conversion of a liquid directly into a gas is called:
a) Evaporation
b) Condensation
c) Sublimation
d) None of the above
Answer: a) EvaporationThe property of a liquid that causes it to rise up a narrow tube against gravity is called:
a) Surface tension
b) Viscosity
c) Capillary action
d) Osmosis
Answer: c) Capillary actionThe process of conversion of a gas directly into a liquid is called:
a) Evaporation
b) Condensation
c) Sublimation
d) Deposition
Answer: b) CondensationThe property of a liquid that causes it to wet the surface of a solid is called:
a) Surface tension
b) Viscosity
c) Capillary action
d) Wetting
Answer: d) WettingThe process of conversion of a solid directly into a liquid is called:
a) Melting
b) Boiling
c) Sublimation
d) Deposition
Answer: a) MeltingThe property of a liquid that determines its resistance to flow is called:
a) Surface tension
b) Viscosity
c) Capillary action
d) Wetting
Answer: b) ViscosityThe temperature at which the vapor pressure of a liquid becomes equal to the atmospheric pressure is called the:
a) Melting point
b) Boiling point
c) Freezing point
d) Critical temperature
Answer: b) Boiling pointThe phenomenon in which a liquid rises up a narrow tube against gravity is called:
a) Osmosis
b) Capillary action
c) Diffusion
d) Adsorption
Answer: b) Capillary actionThe process of conversion of a liquid directly into a solid is called:
a) Melting
b) Boiling
c) Sublimation
d) Freezing
Answer: d) FreezingThe phenomenon in which a solvent moves through a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration is called:
a) Osmosis
b) Capillary action
c) Diffusion
d) Adsorption
Answer: a) Osmosis
- The process of conversion of a liquid into a gas is called:
a) Evaporation
b) Condensation
c) Freezing
d) Sublimation
Answer: a) Evaporation
- The intermolecular forces in gases are:
a) Strongest
b) Weakest
c) Moderate
d) Variable
Answer: b) Weakest
- Which of the following is not a characteristic of solids?
a) Definite shape
b) Definite volume
c) High compressibility
d) Rigid structure
Answer: c) High compressibility
- The temperature at which a liquid starts boiling is called its:
a) Melting point
b) Boiling point
c) Freezing point
d) Sublimation point
Answer: b) Boiling point
- Which of the following is not a physical property of matter?
a) Density
b) Melting point
c) Reactivity
d) Boiling point
Answer: c) Reactivity
- The process of conversion of a solid into a liquid is called:
a) Evaporation
b) Condensation
c) Melting
d) Sublimation
Answer: c) Melting
- The arrangement of particles in solids is:
a) Irregular
b) Random
c) Closely packed
d) Widely spaced
Answer: c) Closely packed
- Which of the following is a chemical change?
a) Melting of ice
b) Boiling of water
c) Dissolving of sugar in water
d) Burning of wood
Answer: d) Burning of wood
- The temperature at which a liquid starts freezing is called its:
a) Melting point
b) Boiling point
c) Freezing point
d) Sublimation point
Answer: c) Freezing point
- The arrangement of particles in liquids is:
a) Irregular
b) Random
c) Closely packed
d) Widely spaced
Answer: b) Random
- The process of conversion of a gas into a liquid is called:
a) Evaporation
b) Condensation
c) Melting
d) Sublimation
Answer: b) Condensation
- The forces of attraction between the particles in solids are:
a) Weakest
b) Strongest
c) Moderate
d) Variable
Answer: b) Strongest
- Which of the following is a physical change?
a) Burning of paper
b) Rusting of iron
c) Dissolution of salt in water
d) Cooking of food
Answer: c) Dissolution of salt in water
- The particles in a gas are:
a) Closely packed
b) Randomly arranged
c) Rigidly held
d) Widely spaced
Answer: d) Widely spaced
- The process of conversion of a solid directly into a gas is called:
a) Evaporation
b) Condensation
c) Melting
d) Sublimation
Answer: d) Sublimation
- The arrangement of particles in gases is:
a) Irregular
b) Random
c) Closely packed
d) Widely spaced
Answer: d) Widely spaced
- The intermolecular forces in liquids are:
a) Strongest
b) Weakest
c) Moderate
d) Variable
Answer: c) Moderate
- The process of conversion of a gas into a solid is called:
a) Evaporation
b) Condensation
c) Deposition
d) Sublimation
Answer: c) Deposition
- The transition of a substance from liquid to gas state is called:
a) Melting
b) Boiling
c) Sublimation
d) Evaporation
Answer: b) Boiling
- The particles in a solid are:
a) Randomly arranged
b) Closely packed
c) Widely spaced
d) Rigidly held
Answer: d) Rigidly held
- The process in which a liquid changes into a gas is called:
a) Boiling
b) Evaporation
c) Condensation
d) Sublimation
Answer: b) Evaporation
- The intermolecular forces in gases are:
a) Strongest
b) Weakest
c) Moderate
d) Variable
Answer: b) Weakest
- The arrangement of particles in a liquid is:
a) Irregular
b) Random
c) Closely packed
d) Widely spaced
Answer: a) Irregular
- The process in which a solid changes directly into a gas is called:
a) Boiling
b) Evaporation
c) Condensation
d) Sublimation
Answer: d) Sublimation
- The particles in a liquid are:
a) Closely packed
b) Rigidly held
c) Widely spaced
d) Randomly arranged
Answer: d) Randomly arranged
- The process in which a gas changes into a liquid is called:
a) Boiling
b) Evaporation
c) Condensation
d) Sublimation
Answer: c) Condensation
- The intermolecular forces in solids are:
a) Strongest
b) Weakest
c) Moderate
d) Variable
Answer: a) Strongest
- The transition of a substance from solid to liquid state is called:
a) Melting
b) Boiling
c) Sublimation
d) Evaporation
Answer: a) Melting
- The process in which a liquid changes directly into a solid is called:
a) Melting
b) Freezing
c) Condensation
d) Sublimation
Answer: b) Freezing
- The arrangement of particles in a solid is:
a) Irregular
b) Random
c) Closely packed
d) Widely spaced
Answer: c) Closely packed
JEE ADVANCE LEVEL MCQS OF MATTER IN OUR SURROUNDINGS
MATTER IN OUR SURROUNDINGS SHORT QUESTIONS
What is matter?
Answer: Matter is anything that has mass and occupies space.Name the three states of matter.
Answer: The three states of matter are solid, liquid, and gas.What is the difference between homogeneous and heterogeneous mixtures?
Answer: Homogeneous mixtures have a uniform composition throughout, while heterogeneous mixtures have a non-uniform composition and can be separated into its components.What is evaporation?
Answer: Evaporation is the process in which a liquid changes into the gaseous state due to the heat energy absorbed by the molecules on the surface of the liquid.Differentiate between boiling and evaporation.
Answer: Boiling is the process in which a liquid changes into the gaseous state due to the heat energy provided to the entire volume of the liquid, while evaporation occurs only at the surface of the liquid.What is diffusion?
Answer: Diffusion is the process by which the particles of a substance tend to spread and mix uniformly throughout another substance due to their kinetic energy.How do intermolecular forces affect the three states of matter?
Answer: Intermolecular forces are responsible for the differences in the three states of matter. In solids, the intermolecular forces are strong, in liquids, they are moderately strong, and in gases, they are very weak.What is sublimation?
Answer: Sublimation is the process in which a solid substance directly changes into the gaseous state without going through the liquid state.What is the importance of the water cycle in nature?
Answer: The water cycle is an important process in nature as it helps in the continuous circulation of water between the Earth's surface and the atmosphere, which is essential for the survival of all living organisms.What is capillary action?
Answer: Capillary action is the ability of a liquid to flow through narrow spaces without the assistance of, and in opposition to, external forces such as gravity.
What is the SI unit of mass?
Answer: The SI unit of mass is kilogram (kg).What is the SI unit of volume?
Answer: The SI unit of volume is cubic meter (m³).
What is the difference between melting and boiling point?
Answer: Melting point is the temperature at which a solid substance changes into a liquid, while boiling point is the temperature at which a liquid substance changes into a gas.What is the role of intermolecular forces in the change of state of matter?
Answer: Intermolecular forces determine the strength of attraction between the particles of a substance, which in turn determines the ease with which the particles can change their positions and arrangement, leading to changes in the state of matter.What is the process of conversion of liquid into gas called?
Answer: The process of conversion of liquid into gas is called vaporization.What is the process of conversion of gas into liquid called?
Answer: The process of conversion of gas into liquid is called condensation.What is the process of conversion of solid into gas called?
Answer: The process of conversion of solid into gas is called sublimation.What is the process of conversion of gas into solid called?
Answer: The process of conversion of gas into solid is called deposition.What are the factors that affect the rate of evaporation?
Answer: The factors that affect the rate of evaporation are temperature, surface area, and humidity.What is the significance of the water cycle in the ecosystem?
Answer: The water cycle is essential for the continuous supply of freshwater, which is necessary for the survival of all living organisms and the maintenance of the ecosystem.
MATTER IN OUR SURROUNDINGS LONG QUESTIONS
Question: Describe the three states of matter and explain the differences in their properties.
Answer: The three states of matter are solid, liquid, and gas. In the solid state, the particles (atoms, molecules, or ions) are closely packed and have a fixed arrangement, resulting in a definite shape and volume. Liquids have a definite volume but no definite shape, as the particles have more freedom to move. Gases have neither a definite shape nor volume, as the particles are widely spaced apart and have the highest freedom of movement. The key differences between the three states lie in the strength of the intermolecular forces, the compressibility, and the ability to maintain a defined shape or volume. These properties vary significantly, with solids being the most rigid and least compressible, while gases are the most compressible and lack a defined shape or volume.
Question: Explain the changes that occur during the interconversion of the three states of matter (solid, liquid, and gas). Describe the factors that affect the rate of these changes.
Answer: The interconversion of the three states of matter involves changes in the arrangement and movement of the constituent particles (atoms, molecules, or ions). During melting, the particles in a solid gain enough energy to overcome the strong intermolecular forces, allowing them to move more freely and transition to the liquid state. Boiling occurs when the particles in a liquid gain sufficient energy to escape the liquid phase and enter the gaseous state. Conversely, condensation involves the loss of energy by gaseous particles, leading to the formation of a liquid, while freezing is the transition from liquid to solid as the particles lose energy and their movement becomes more restricted. The rate of these state changes is influenced by factors such as temperature, pressure, surface area, and the presence of catalysts or impurities, which can either facilitate or hinder the required energy changes and the overcoming of intermolecular forces.
Question: Describe the concept of diffusion and explain its importance in daily life. What factors affect the rate of diffusion?
Answer: Diffusion is the spontaneous movement of particles (atoms, molecules, or ions) from a region of higher concentration to a region of lower concentration, driven by the random thermal motion of the particles. This process occurs in all three states of matter and plays a crucial role in various everyday phenomena, such as the spread of fragrances, the mixing of substances in solutions, and the transport of essential gases like oxygen and carbon dioxide in living organisms. The rate of diffusion is influenced by several factors, including the size and mass of the diffusing particles, the temperature of the system, and the viscosity of the medium. Smaller and lighter particles generally diffuse faster, as do systems with higher temperatures and less viscous media. Understanding the principles of diffusion is essential in fields like biology, chemistry, and engineering, where it is used to optimize various processes and improve the efficiency of systems.
